
GUEST BLOG POST
Lately, I’ve been reflecting on my experience presenting compliance updates to boards, both during my industry days and now as a consultant. One thing that consistently frustrates me is seeing compliance officers deliver presentations that are completely reactive. The focus is always on the number of investigations closed, training completion percentages, number of policies approved, auditing and monitoring results, and similar reports. Frankly, it’s easy for board members’ eyes to glaze over with this approach.
While these elements are important for the board to understand, the actual compliance presentation at board meetings often misses the mark by failing to showcase the proactive work that a compliance team is doing. Compliance officers are often not effectively demonstrating how they are aligned with the evolving and innovative strategies of their business, industry, and environment.
Compliance officers occupy a unique vantage point in their companies. They have unparalleled visibility into almost every facet of an organization’s operations. This allows them to understand the workings and interplay between technology, ever-evolving regulations, and day-to-day business practices. In my experience, the most engaging board presentations are the ones where the compliance officer can articulate what the compliance department is proactively doing to address emerging phenomena, discussing both the risks and the mitigation strategies in place. It positions the compliance officer as a strategic partner, not one who impedes progress.
This proactive approach not only progresses the compliance agenda at the highest levels of the organization, it also directly aligns with the expectations of the U.S. Department of Health and Human Services – Office of Inspector General (HHS-OIG), Department of Justice (DOJ), Securities and Exchange Commission (SEC), and other relevant regulators.
Next, we’ll consider five key topics compliance officers should be actively discussing with their boards in 2024. We’ll explore how to move beyond reactive reporting and demonstrate your role as a strategic partner. While we’ll focus on the life sciences sector, many of the topics are relevant to all compliance functions.
1 Digital Enablement
Digital enablement continued to increase in importance during in the first six months of 2024. Artificial Intelligence and Machine Learning (AI/ML) are revolutionizing drug development and clinical trials by enabling the analysis of vast amounts of data and accelerating the discovery of new treatments. AI/ML algorithms can identify patterns and predict outcomes, aiding in the selection of potential drug candidates and predicting patient response to treatments. By optimizing trial design, AI/ML can improve the efficiency of clinical trials, leading to faster and more accurate results. Outside the life sciences sector, AI is quickly inhabiting nearly every aspect of the organization, raising endless possibilities for innovation and efficiency, while also unveiling several complex risks.
Drug Discovery
- AI/ML algorithms are being used to analyze vast amounts of data from genomics, proteomics, and other sources to identify potential drug candidates and predict their efficacy and safety.
Clinical Trial Design
- AI/ML can be used to optimize clinical trial design, such as identifying the most appropriate patient population, optimal dosing levels, and predicting potential adverse events.
Trial Data Analysis
- AI/ML can be used to analyze clinical trial data more efficiently and identify potential safety signals or trends, allowing for faster course correction and improved drug development outcomes.
Similarly, AI/ML is transforming the way nearly all companies approach commercial activities. Using predictive analytics, AI/ML can assist companies in identifying potential customers, creating personalized marketing strategies, and predicting future market trends.
Content Personalization
- AI can generate personalized marketing materials, such as email content, website landing pages, and social media posts, tailored to the specific needs and interests of customers and other stakeholders.
Sales Optimization
- AI can analyze sales data with healthcare professionals (HCPs) and Healthcare Organizations (HCOs) to prioritize them based on likelihood of Rx conversion, helping sales teams focus their efforts on the most promising opportunities.
Sentiment Analysis
- AI can analyze patient and caregiver feedback and social media conversations to identify trends and potential issues, allowing for proactive customer service and reputation management.
Action Items: Compliance officers should be proactive in establishing robust data governance policies, collaborating with the AI/ML team to mitigate potential algorithmic bias, and working across the company to develop a comprehensive compliance framework for AI/ML use. When communicating with the board, keep them informed about how you are tracking with the company’s AI/ML initiatives, highlighting the potential benefits and associated risks. Discuss the steps your compliance team is taking to mitigate these risks, including partnering on data governance policies, bias mitigation strategies, and adherence to regulatory frameworks.
2 The Talent Shuffle
The life sciences industry in 2024 presents a tale of two realities. While a wave of innovation is fueling growth for some, established players are resorting to cost-cutting measures, leading some companies to institute major layoffs. These same forces are impacting companies in just about every industry.
Cost Cutting: Life sciences companies often face the need to reduce costs to remain competitive. We’ve seen several announcements thus far this year:
- Pfizer – $4 billion cost-cutting by end of 2024 + $1.5 billion over next 3 years
- Bristol Myers Squibb – 2,000 employees impacted by layoffs
- Bayer – reduced headcount by 1,500 employees
- Takeda – 641 workers impacted by layoffs
Talent Retention: Retaining talented employees contributes to the long-term success of the company. Companies are using a variety of mechanisms to attract and retain talent. These include: highlighting the company’s unique mission and culture; innovative compensation models; hybrid work arrangements; upskilling programs; wellbeing offerings; Diversity, Equity, and Inclusion (DEI) focus; and commitment to career development.
Depending on the stage of a company’s product lifecycle and market, different strategies may be implemented. Some life sciences companies may focus on cost-cutting, while others prioritize talent retention. In certain cases, companies may simultaneously pursue both objectives.
Action Items: Compliance officers need to be proactive as the employee landscape shifts. With new hires and role changes, a crucial focus should be on providing targeted training and education on role-specific compliance requirements. However, this isn’t the only concern. Compliance officers should also identify areas where existing controls may become inadequate or even disappear entirely due to staffing changes. The compliance officer should inform the board about these potential control gaps and propose solutions, such as increased monitoring or adjustments to existing processes and controls. More importantly, these changes may necessitate a revision of the company’s risk assessment. If key personnel with deep operational and compliance knowledge depart or controls are weakened, the overall risk profile of the company can shift significantly. The compliance officer should work with relevant departments to re-evaluate the risks, identify new vulnerabilities, and update the risk assessment accordingly.
3Decentralized Clinical Trials
Decentralized Clinical Trials (DCTs) are a growing trend in the pharmaceutical industry. These trials leverage technology to collect data remotely, reducing the need for in-person visits. This allows for greater patient participation, especially from geographically dispersed populations or those with mobility limitations. Examples include telehealth-based trials using video conferencing, wearable devices collecting health data like heart rate and activity levels, and mobile apps for patient-reported outcomes and communication.
However, DCTs also raise compliance concerns. Data security and privacy require robust security measures, clear data governance policies, and strong encryption protocols. Patient privacy is another consideration, as remote data collection necessitates carefully adapted informed consent procedures to address potential coercion or undue influence. Finally, regulatory bodies are still developing guidelines for DCTs, creating some uncertainty for companies.
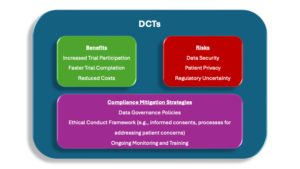 Action Items: To navigate the evolving DCT landscape, compliance officers must stay informed about changing regulations and develop clear policies for ethical conduct in DCTs. This includes adapting informed consent procedures for the remote setting, implementing robust patient data protection protocols, and establishing clear communication channels to address patient concerns. Compliance officers should be proactively informing their boards on how the compliance program is helping the company leverage the benefits of DCTs while minimizing risks and maintaining ethical practices.
Action Items: To navigate the evolving DCT landscape, compliance officers must stay informed about changing regulations and develop clear policies for ethical conduct in DCTs. This includes adapting informed consent procedures for the remote setting, implementing robust patient data protection protocols, and establishing clear communication channels to address patient concerns. Compliance officers should be proactively informing their boards on how the compliance program is helping the company leverage the benefits of DCTs while minimizing risks and maintaining ethical practices.
4ESG Considerations
Environmental, Social, and Governance (ESG) factors continue to remain important for investors and stakeholders. Boards are discussing how to integrate ESG principles into their corporate strategy and demonstrate their commitment to sustainability and social responsibility. Boards are facing challenges in this space.
Lack of Standardized Regulations
- Currently, there’s no single, overarching set of ESG regulations globally. Different countries have varying regulations and reporting and disclosure requirements, making it complex for companies with international operations.
- Action Item: Compliance officers must stay updated on these diverse regulations to ensure adherence across all markets.
Greenwashing Concerns
- Regulatory bodies are increasingly scrutinizing ESG claims to prevent “greenwashing,” where exaggerated information is presented about a company’s sustainability efforts.
- Action Item: Compliance officers should be working cross-functionally and sharing with the board how the company’s is ensuring its ESG reporting is accurate, transparent, and verifiable to avoid potential penalties and reputational damage.
Consumer Protection
- Consumer protection regulations are evolving to address misleading environmental claims in marketing.
- Action Item: Compliance officers must collaborate with commercial teams, corporate affairs, and their PRC committees to ensure all ESG-related messaging is accurate and substantiated.
Cybersecurity Risks
- The increasing collection and use of ESG data introduces new cybersecurity risks.
- Action Item: Compliance officers need to work with IT and other groups gathering data in the organization to implement policies and robust data security measures to protect sensitive ESG information from breaches or misuse.
5 Economic and Geopolitical Headwinds
The life sciences industry is continuing to face several disruptive macro forces in 2024. Beyond the ongoing challenges of scientific advancement and regulatory compliance, boards of directors are grappling with a complex economic and geopolitical landscape. This is across all industries, not just life sciences. The war in Ukraine, ongoing tensions between major powers, and escalation in the Israeli-Palestinian conflict are creating significant supply chain disruptions, potentially impacting research collaborations and access to critical resources. Coupled with a persistent inflationary environment, boards are strategizing on how to navigate these economic headwinds. This could involve cost-cutting measures (previously explored), investigating alternative sourcing options, or even raising prices to maintain profitability.
Action Items: For compliance officers, these disruptions present unique challenges. Inflationary pressures may incentivize corners being cut, potentially impacting quality control measures or adherence to Good Manufacturing Practices (GMP). Compliance officers should be informing the board about potential risks associated with cost-cutting measures, as well as the potential legal and reputational consequences of non-compliance. Additionally, compliance officers should be prepared to advise the board on navigating the complexities of a shifting geopolitical landscape. This could involve ensuring robust due diligence on new suppliers and research partners, mitigating the risk of sanctions violations, and helping the business ensure continued access to critical resources.
From Reactionary to Proactive
Compliance officers have a golden opportunity to continue to transform their role. By proactively tackling the aforementioned topics and demonstrating a strategic grasp of the industry’s evolving landscape, they can become invaluable partners to their boards. This shift transcends mere reporting. Instead of simply reacting to events, compliance officers can anticipate risks, propose solutions, and actively align with the company’s strategic goals. This proactive approach will only strengthen their compliance program.
Key Takeaways
- Compliance officers must align with board priorities to truly become a strategic partner.
- Compliance officers should discuss with the board how they are helping mitigate digital enablement risks, including partnering on data governance, adherence to regulatory frameworks, and bias mitigation strategies.
- High turnover weakens controls, raising risk. When the employee landscape shifts, compliance officers need to identify gaps and refresh risk assessments.
- Compliance officers need to ensure their programs are adapting for decentralized clinical trials (DCTs).
- Compliance officers must continue to advise the board on responsible ESG reporting and navigating sanctions and supply chain risks.

Amy Pawloski, CCEP, CFE, PMP (amy.pawloski@strategicversatility.com) is the president of Strategic Versatility LLC a healthcare compliance consulting practice in Phoenixville, Pennsylvania.

